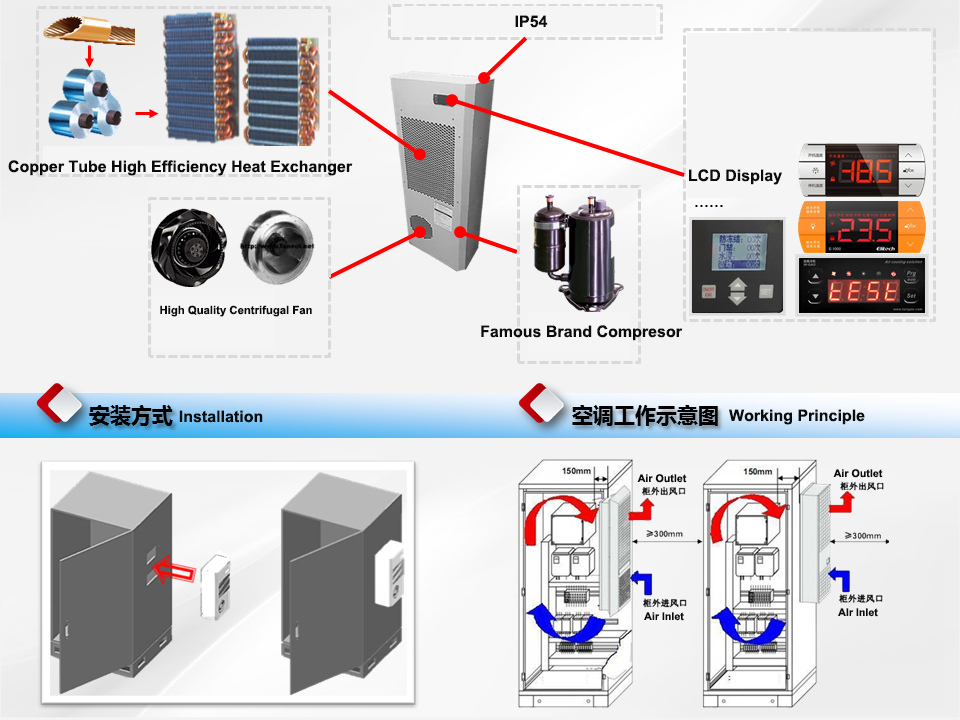
Side Mounted Cabinet Air Conditioner
This type Cabinet Air Conditioner are installed on the side of the cabinent / enclosure.
Cooling Capacity is from 300W to 8000W
Poduct Features:
Water-proof, Dust-proof: Well Sealed design and air filter instrument realize IP 54 efficient protection, IP55 also available
Precise Temperature and Humidity Control: Use high quality components to control temperature and humidity to ensure the best working condition inside the cabinet.
Anti-corrosion: Surface of the cabinet are made of aluminum alloy, which can protect from UV and salt material.
Normal wall-mounted cabinet air conditioner Side Mounted Cabinet Air Conditioner,Side Mounted Air Conditioner,Electrical Panel Air Conditioner,Side Mounted Control Cabinet Air Conditioner Taizhou Tentcool Electrical Appliance Co., Ltd. , https://www.tentcool.com








Complex multi-metal sulfide gold ore is an important type of gold resources, I domestic gold base in Shandong, Henan and other provinces storage of large quantities of these ore, copper, Jiangxi, Anhui, Hunan and other copper base in the Yangtze River region commonly associated gold. Such ores are mainly sulfides and sulfide-depleted or gold - pyrite type gold - copper - pyrite, gold - Quartz - multi-metal type. In addition to gold is intimately associated with pyrite, the mostly copper, lead and other minerals intimately associated. The problem arising from the gold extraction of gold ore is directly related to the occurrence of gold in the ore and the carrier mineral. The gold-copper sulfide ore type gold deposit is the main type and is also a common refractory ore. Such ores are directly cyanated and leached, generally have a low leaching rate and consume a large amount of cyanide. The main reasons for its difficulty in immersion are as follows: First, metal sulfides such as copper, iron, bismuth , manganese and nickel in complex polymetallic sulphide ore-type gold minerals are easily chemically reacted with oxygen in the air in the immersion liquid, and consume a large amount. Oxygen and alkali. At the same time, these metal ions can react with cyanide ions. For example, copper and cyanide ions can form various copper complexes according to the concentration ratio of cyanide to copper in solution. For example: Cu(CN), Cu(CN 2 - , Cu(CN) 3 2 - , Cu(CN) 4 3 - , consume a large amount of cyanide ions. Second, the dissolution of copper and other base metal sulfide ore in the immersion liquid not only consumes oxygen and cyanide ions, but the oxidation product can form a passivation film on the surface of the gold particles, or react with cyanide to form an insoluble compound covering the gold particles, and Lowering the potential of the immersion liquid reduces the cyanidation rate of gold or cyanidation. The third is copper, iron, antimony, manganese, nickel, lead and other metal ions in complex polymetallic sulfide ore-type gold minerals, which can form cyanide ions with cyanide ions and copper cyanide ions into solution. substitution gold zinc powder, ion exchange, activated carbon adsorption and solvent Cuiqu have adverse effects. Therefore, such ore needs to be pretreated to remove copper, iron, bismuth and other metals and then leached with cyanide or other leaching agent. At present, the pretreatment methods mainly include calcination oxidation method, bacterial oxidation method, pressure oxidation method, chemical oxidation method and the like.
In this paper, pure copper sulfide minerals were used as research objects. In the acidic system with added chlorine salt, the study of warming and pressurized pre-oxidation leaching and copper removal was carried out, aiming at the industrial application of practical copper-containing refractory gold ore and difficult to be beneficiated. The theoretical application of the wet processing industrial application of enriched low-grade copper sulfide ore provides a theoretical basis.
1. Samples, reagents and research methods
(a) sample
The well-crystallized chalcopyrite is taken from a copper mine. The pure minerals are selected by hand. The chalcopyrite minerals are crushed, ground and sieved and washed repeatedly with distilled water. After drying, the bottles are reserved. After testing, the sample contained 33.25% copper and the purity was over 95%.
(2) Main pharmaceuticals and instruments
The main reagents used in the test were concentrated sulfuric acid, sodium chloride and oxygen, which were produced by domestic chemical pharmaceutical factories. Concentrated sulfuric acid and sodium chloride were analytically pure and oxidized to industrial purity. The main equipment used in the test was titanium -lined FCN type 2L autoclave for pressurized pre-oxidation, XL-30W/TMD type scanning electron microscope, EDAX type spectrometer for surface structure analysis of leaching slag, miniflex type and X-ray diffractometer For the analysis of the leaching slag phase, the 80TDE ultrasonic cleaner is used to clean the surface of the leaching slag.
(3) Research methods
The oxidation pretreatment test was carried out in a FCH type 2L titanium-filled autoclave. After the ore is ground to a suitable particle size in a mill, it is adjusted in a beaker according to the test conditions, and then added to the autoclave. According to the test conditions, adjust the stirring speed, add oxygen in time, adjust the oxygen partial pressure of the autoclave, maintain the pressure balance of the autoclave, and keep the temperature inside the kettle. After the pressure oxidation treatment, the filter is filtered in a multi-purpose vacuum filter, the liquid is sent to the test, and after the slag is washed and dried, part of the sample is sent for analysis and analysis, and part is used for testing. The phase analysis was performed using a miniflex type and an X-ray diffractometer.
Second, the test and results
(1) Influence of main factors of leaching process on copper and iron in pre-oxidized leaching of chalcopyrite
During the process of pressurized pre-oxidation leaching, the main process parameters such as oxygen partial pressure, temperature, initial sulfuric acid concentration and initial sodium chloride concentration have an important influence on the pre-oxidation of copper and iron in chalcopyrite. Figure 1 shows -45μm particle size 80%, leaching temperature 110 °C, initial H 2 SO 4 concentration 0.37mol / L, initial NaCl concentration 0.68mol / L, liquid-solid ratio 20:1, leaching time 80min, stirring speed 750r / The effect of partial pressure of oxygen on the oxidative leaching rate of copper and iron under pre-oxidation conditions of min. The results in Figure 1 show that as the partial pressure of oxygen increases, the leaching rate of copper increases, and the leaching of iron decreases with the increase of oxygen partial pressure. Therefore, correspondingly increasing the partial pressure of oxygen is advantageous for the oxidative pretreatment effect. However, high oxygen pressure is not conducive to industrial production, and reducing oxygen pressure is also the purpose of research. When the oxygen pressure is 0.45Mpa, the leaching rate of copper has reached 84.68%. When it is increased to 0.55MPa, the copper leaching rate is increased to 85.01%. Therefore, it is suitable to use an oxygen pressure of 0.45 MPa.
Fig.1 Effect of oxygen partial pressure on copper and iron in pre-oxidized leaching chalcopyrite
Figure 2 shows the ore sample -45μm particle size 80%, oxygen partial pressure 0.45MPa, initial H 2 SO 4 concentration 0.37mol / L, initial NaCl concentration 0.68mol / L, liquid-solid ratio 20:1, leaching time 80min, stirring The effect of temperature on the oxidative leaching rate of copper and iron in chalcopyrite at a speed of 750r/min. The results in Figure 2 show that the temperature has a great influence on the leaching rate of copper and iron in chalcopyrite. In the range of 90 °C to 110 °C, the copper leaching rate increases rapidly with the increase of temperature, and the leaching rate of iron rises first and then falls. From 110 ° C to 120 ° C, with the increase of temperature, the leaching rate of copper increased little, and the leaching rate of iron decreased significantly. Considering that 119 ° C is the melting point of elemental sulfur, close to the melting point of sulfur is not conducive to the leaching of chalcopyrite, and cyanidation after pretreatment, therefore, 110 ° C is suitable.
Fig. 2 Effect of temperature on copper and iron in pre-oxidized leaching chalcopyrite
Figure 3 shows the temperature of 110 ° C, -45 μm level of 80%, oxygen partial pressure of 0.45MPa, initial NaCl concentration of 0.68mol / L, liquid-solid ratio of 20:1, leaching time of 80min, stirring speed of 750r / min, from The effect of acidity on the leaching rate of copper and iron in chalcopyrite. It can be seen from Fig. 3 that the amount of sulfuric acid is less than 0.37 mol/L, the leaching rate of copper increases with the increase of acidity, and the amount of sulfuric acid is more than 0.37 mol/L. The leaching rate of copper decreases with the increase of acidity. When the amount of H 2 SO 4 is less than 0.55 mol/L, the leaching rate of iron increases remarkably with the increase of acidity, but when the amount of H 2 SO 4 is higher than 0.55 mol/L, the leaching of iron begins to decrease. It can be seen that the optimum initial concentration of H 2 SO 4 is 0.37 mol/L.
Fig. 3 Effect of sulfuric acid dosage on copper and iron in pre-oxidized leaching chalcopyrite
Figure 4 shows the temperature of 110 ° C, -45 μm particle size accounted for 80%, oxygen partial pressure of 0.45MPa, initial H 2 SO 4 concentration of 0.37mol / L, liquid-solid ratio of 20:1, leaching time of 80min, stirring speed of 750r / min Under the condition, the effect of NaCl concentration on the leaching rate of copper and iron in chalcopyrite. The results in Figure 4 show that when the amount of NaCl is low, the leaching rate of copper is extremely low. With the increase of NaCl concentration, the leaching rate of copper increased rapidly, and the leaching rate of iron decreased rapidly and then increased a little. It is obvious that the influence of NaCl on chalcopyrite leaching is more complicated. However, when the concentration of sodium chloride is higher than 0.68 mol/L, the effect on the leaching of copper and iron is not. Therefore, it is appropriate to determine the initial NaCl concentration to be 0.68 mol/L.
Figure 4 Effect of NaCl concentration on copper and iron in pre-oxidized leached chalcopyrite
(II) Pressurized pre-oxidation leaching test of a polymetallic sulphide ore type copper-bearing gold ore
On the basis of the single factor condition test, a pressurized pre-oxidation leaching test was carried out on a polymetallic sulphide-type copper-bearing gold ore containing 20% ​​copper and 20 g/t gold. The leaching conditions are 100g of copper-bearing gold ore, 85% of -45μm, liquid-solid ratio of 5:1, initial sulfuric acid concentration of 0.55mol/L, sodium chloride concentration of 0.68mol/L, leaching time of 2.5h, temperature of 110°C. The test results are shown in Table 1. The results in Table 1 show that when the partial pressure of oxygen reaches 0.45 MPa, the gold leaching rate can reach 96.35% or more.
Table 1 Test results of pressurized pre-oxidation leaching of a polymetallic sulphide ore type copper-bearing gold ore
Oxygen partial pressure / MPa
Leaching residue Cu content /%
Leaching slag Fe content /%
Cu leaching rate /%
Fe leaching rate /%
Au leaching rate /%
0.55
1.30
27.97
93.30
33.62
97.43
0.45
2.64
27.74
87.80
31.98
96.35
Third, the pre-oxidation leaching chemical reaction process of chalcopyrite
The chemical analysis of the pre-oxidized leachate under different conditions showed that Fe was mainly oxidized to Fe 2+ and a small amount of Fe 3+ was present in the leaching liquid at the initial temperature of 90-120 °C. At high temperature and in the late stage of oxidative leaching, Fe is mainly precipitated in the leaching residue by trivalent iron bismuth, and some Fe 3+ and a small amount of Fe 2+ are present in the leaching liquid. Copper is present in the leaching solution with Cu 2+ and CuCl 2 - . When the potential of the leaching system is high, the final product of copper oxidation in the solution is Cu 2+ . Therefore, it can be considered that the final product in the leaching solution of the oxidized and leached pure chalcopyrite by the warmed pressurized hydrochloric acid system is Cu 2+ , Fe 3+ ions and various products formed thereof with chloride ions. Based on the chemical analysis of the pre-oxidized leaching solution under different conditions, the X-ray diffraction analysis of the pre-oxidized leaching slag under different conditions was carried out, and the results are shown in Figures 5-7. Figure 5 is an X-ray diffraction diagram of pure chalcopyrite. Figures 6 and 7 show pure brass at a temperature of 110 ° C, an oxygen partial pressure of 0.45 MPa, an initial sulfuric acid concentration of 0.55 mol/L, and a sodium chloride concentration of 0.68 mol/L. The X-diffraction pattern of mineral pre-oxidation for 10 min and 80 min can be seen from the figure. At lower temperature or shorter time, the slag is mainly unreacted chalcopyrite. As the oxidation progresses further, the sulphur content in the slag gradually Elevated, while the precipitation of iron steroids also increases with the progress of the reaction. Figure 6 shows that when pre-oxidized for 10 min, the leached iron ions have begun to precipitate in the leaching slag with ferric sulphate; Figure 7 shows that with the oxidization time prolonged, the leaching is deep, the yellow samarium strontium The amount of precipitation increases. At the same time, with the increase of pH, the precipitation of sassafras was initiated. It indicates that the pre-oxidation is more complete with the increase of oxidation time. In the oxidation process of chalcopyrite, firstly, iron is preferentially separated from the chalcopyrite crystal lattice, and many intermediate products such as Cu 9 Fe 9 S 16 , Cu 39 S 28 , CuCl and the like are formed. During the oxidation process, Cu 9 Fe 9 S 16 , Cu 39 S 28 and yellow sodium iron sputum and grass yellow iron sputum are formed, while yellow sodium iron sputum and grass yellow iron sputum are the final products of iron. Therefore, it can be considered that the total reaction of oxidative leaching of chalcopyrite in a chlorinated hydrochloric acid system under heating and pressurization is:
4CuFeS 2 +10H 2 SO 4 +5O 2 =
4CuSO 4 +2Fe 2 (SO 4 ) 3 +8S o +10H 2 O
The ferric iron is further reacted to form yellow sodium iron strontium, and the molecular formula is Na x (H 2 O) 1 - x [Fe 3 (SO 4 ) 2 (OH) 6 ].
Figure 5 Pure chalcopyrite X diffraction pattern
Figure 6 X-ray diffraction pattern of leaching slag for 10 min oxidation
Fig. 7 X-diffraction pattern of leaching slag oxidized for 80 min (slag after scouring a large amount of elemental sulfur)
In the process of pre-oxidizing and leaching chalcopyrite using a low-temperature, low-pressure chlorine-hydrochloric acid system, elemental sulfur is a reaction product that is desired to be formed, and the formation of elemental sulfur minimizes oxygen consumption. However, the elemental sulfur produced cannot be encapsulated in gold, otherwise it will be detrimental to cyanide leaching. Tests have shown that in a chlorine-hydrochloric acid system with an oxidation temperature of less than 110 ° C, when the sulfuric acid concentration is less than 0.55 mol / L, the sulfur oxidation product in chalcopyrite is basically elemental sulfur, see Figure 7. The calculations show that the sulfur oxidized to sulfuric acid is almost zero except for the copper oxide compound which is not completely oxidized. This is consistent with the results of some literatures showing that the oxidation product of sulfide ore in the oxidation system at 120 °C is mainly elemental sulfur. of. The elemental sulfur is very dispersed, does not reunite with other solid slag, and is easily separated by slightly washing with water. From the scanning electron micrograph of elemental sulfur in the leaching slag, the appearance of elemental sulfur can be clearly seen. The fine elemental sulfur particles agglomerate to each other as small particles of several tens of micrometers, and the surface has many small pores, showing a loose structure.
Figure 8 SEM image of elemental sulfur in leaching residue
Fourth, the conclusion
(1) The experiment of oxidizing and leaching pure chalcopyrite with chlorinated salt in the pressurized and pressurized acid system has investigated the effects of oxygen partial pressure, initial acidity, initial NaCl concentration and temperature on the oxidation and leaching of copper and iron. The results show that sulfuric acid concentration, sodium chloride concentration, temperature and oxygen pressure are important factors affecting the leaching of chalcopyrite. Suitable sulfuric acid concentration, sodium chloride concentration, sodium chloride concentration, temperature and oxygen pressure are beneficial to chalcopyrite. Pre-oxidation leaching. However, the impact of various factors on iron leaching is more complicated.
(2) Under the conditions of temperature 110 ° C, oxygen partial pressure 0.45 MPa, oxidation time 2.5 h, mineral particle size -44 μm 85%, initial acidity 0.55 mol/L, initial NaCl concentration 0.68 mol/L, The warming and pressurized pre-oxidation leaching of copper-gold ore obtained 96.35% pre-oxidation leaching rate of copper, indicating that the process can oxidize sulfide ore and remove copper and other metals. The process is complex copper-bearing gold ore with complex polymetallic sulfide ore. Pre-oxidation treatment is technically feasible.
(III) X-ray diffraction analysis of pre-oxidized leaching slag under different conditions and scanning electron microscopy analysis of elemental sulfur in leaching slag showed that the pre-oxidation of chalcopyrite was more complete with the prolonged oxidation time under suitable pre-oxidation conditions. The elemental sulfur formed by the pre-oxidation slag presents a loose structure.