It represents metal leaching rate versus time function, related to the rate of mass transfer of reactants or chemical reaction rate of the solid surface. But for the purpose of determining process control under conditions, the most convenient way is to use a mathematical equation to fit the curve without mentioning these factors. This advancement and retreat of the surface affects the leaching kinetics as the surface of the solid reactant in a leaching system advances or retreats as the reaction progresses. This is also the case when solid reaction products are continuously formed around the reactive ions. Based on this behavior, mathematical equations for several models were obtained and used to describe the shape of the rate curve obtained by leaching minerals under different conditions. The chemical behavior of the leaching system is extremely important when determining the chemical conditions used, especially when developing new processes. First, the shrinkage particle model Exporting a contracted particle model assumes that the particle is a sphere, but the resulting final equation can be applied to any other shape of equal volume particle. Let the number of moles n of unreacted spheres be n=4Ï€r 3 ∕3V (1) Where V-molar volume is equal to m∕Ï, where m is the mass and Ï is the solid density. The reaction rate of the surface of the sphere with radius r can be written as -dn∕dt=4Ï€r 2 Ch' (2) Where k' is the first order reaction rate constant of the reactant at a concentration of C in the solution. The equation (1) is differentiated from time and substituted into equation (2) to obtain a linear rate -dr∕dt=VCk' (3) Where VCk' is the linear rate constant k 1 . If C is the concentration of the reaction solute expressed in mol ∕ cm 3 , the linear rate at which the radius r decreases is expressed in cm ∕ s. If C is left unchanged, equation (3) represents a constant rate of movement of the reaction interface, which is the definition of linear dynamics. If the initial radius of the reactive particles is r 0 and α is the reaction score, then The above formula is different for time Combine equations (3), (4), and (5) For the initial condition t=0, α=0, it can be assumed that C is constant and the integral of equation (6) is obtained. Where k = Ck 1 ∕r 0 (time - 1 ). To plot the left side of equation (7) against t, we should get a straight line with a slope of k, and the dimension of k is 1∕t. The above model is derived from a single particle. If all the ore particles in the slurry have the same initial diameter, the reaction combination rate of many ore particles will also obey this equation. For the slurry with a certain particle size distribution, the mass fraction w i of each particle size distribution must be known. Assuming that the initial average ore radius of the mass fraction w i is r i0 , then equation (8) becomes Where α i is the reacted fraction with a mass fraction of w i . The total amount of reaction solids is α= If the concentration changes as the reaction progresses, the change in concentration must be considered in the integral basic rate expression. For surface control reactions, or for diffusion through a boundary film of the limit, equation (9) must account for the concentration of the reactants, thus obtaining Wherein C 0 - initial concentration; Σ-stoichiometric coefficient; b = n 0 ∕V 0 C 0 , C 0 , n 0 are the total moles of minerals in the system, respectively. In the specific case of σb=1, the integral is obtained. Second, the shrinking core model In many cases mineral particles contain a variety of metals, while only one metal is eluted when leaching. This situation can result in the formation of a porous solid reaction product around each of the ore particles that are reacting. Only one special case is considered here, that is, the particle radius always remains equal to the original radius r 0 of the particle before the reaction starts. The shrinking core of radius r continues to react at a rate determined by the rate at which the reactants diffuse through the product layer toward the reaction interface. If the reaction particles are spherical, the reaction rate can be written as: The number of moles of unreacted mineral in the n-core; Σ-metering factor; C- the number of moles of diffusing material required to leach 1 mole of metal from the core; The effective diffusion coefficient in the D-product layer is the concentration of the reactants at the interface of the shrinking core. In the steady state condition, from r to r 0 integral, when the concentration C of the reactant on the interface is much smaller than the bulk concentration C 0 , The equations for combining the equations (12) and (13) to obtain the core and reaction product boundary movement rate expressed by the radius r of the unreacted core are as follows Combining equations (14), (4) and (5), obtains a reaction rate equation expressed by the fractional α of the reaction. When the boundary condition t=0, α=0, the integral is obtained. The left side of the above equation is plotted against time to get a straight money, and the slope is proportional to r 0 -2 . Similarly, when it is necessary to consider the change in the concentration of the reactants, the equation (15) becomes a diffusion through the product or the residual layer. In the specific case of σb=1, the integral is obtained. In the general case of σb ≠1, equations (13) and (17) need to be solved by numerical integration.
BRIGHT STEEL BAR
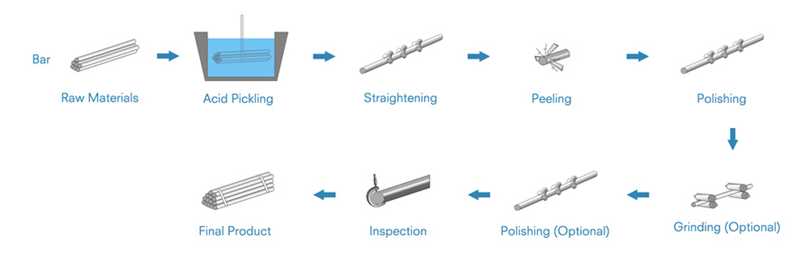
Bright steel bar is carbon or alloy steel bar with shining bright surface which is improved by cold drawing, peeling, turning, grinding and polishing over hot rolled black surface bars. By cold drawing, the steel bar yield strength and tensile strength will be improved. By peeling and turning, the surface imperfections will be removed. By grinding, the diameter tolerance will be more precision. By polishing, the surface will be much more shining.
Except the shining bright surface, comparing with the hot rolled black surface bars, the bright steel bars has better surface roughness, better roundness, straightness, more precision size tolerance etc.
Heat treatment such as annealing, normalizing, quenching and tempering will be done before do cold finishing on the hot rolled bar to get bright steel bar, in such condition, the steel bright bar will get most suitable mechanical properties to meet different application requirement.
The advantages of steel bright bars we supply:
1) Big stocks of hot rolled round bars or wire rods as raw materials
2) Heat treating furnaces to adjust the mechanical properties
3) Full sets of testing equipment to test the sizes, mechanical properties and microstructure.
4) Multiple packages to avoid broken packages and anti-rusty
Diameter tolerance
+0/-0.02mm
Straightness
0.5mm/m
Surface roughness
0.4um
Roundness
80% of diameter tolerance
Diameter range
14mm to 100mm
Steel grade
Kinds of carbon steel and alloy steel
Length
Any length can be cut with precision tolerance
Bright Steel Bar,Bright Steel Round Bar,Steel Bright Bar,Bright Polished Steel Bar,Alloy Steel Bright Bar Manufacturer SHANDONG LE REN SPECIAL STEEL CO., LTD. , https://www.sdhighstrengthsteelbolts.com![]() (4)
(4) ![]() (5)
(5) ![]() (6)
(6) ![]() (7)
(7) ![]() (8)
(8) ![]() (9)
(9) ![]() .
. ![]() (10)
(10) ![]() (11)
(11) ![]() (12)
(12) ![]() (13)
(13) ![]() (14)
(14) ![]() (15)
(15) ![]() (16)
(16)  (17)
(17) ![]() (18)
(18)